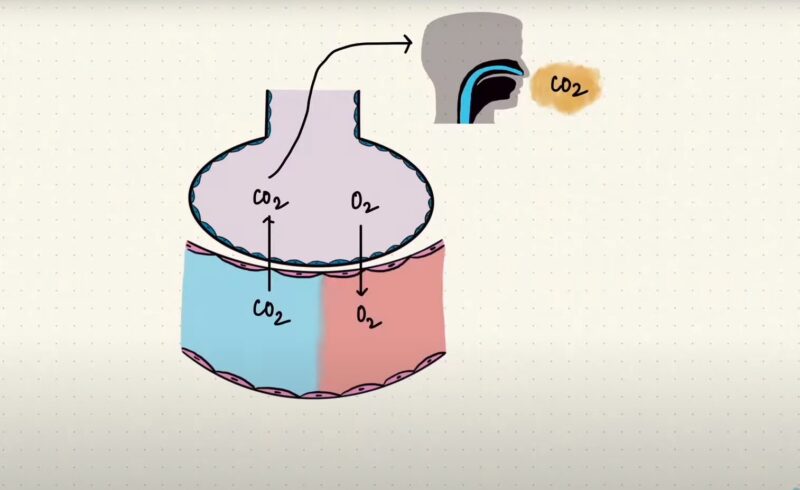
The human body is truly incredible, designed with intricate biological mechanisms that work together seamlessly to ensure our survival and well-being. One of these remarkable processes involves the transportation of oxygen from our lungs to every nook and cranny of our body.
Playing a crucial role in this process is a special protein called hemoglobin, which resides within our red blood cells. However, it’s fascinating to discover that the efficiency of oxygen transport is not fixed and can be affected by a multitude of factors. This is where the Bohr Effect steps in, lending a hand in regulating this intricate dance of oxygen delivery.
What is the Bohr Effect?
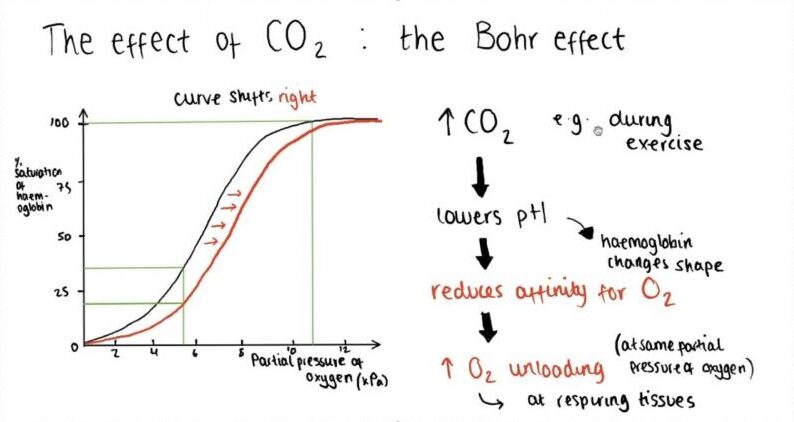
The Bohr Effect, named after the Danish physiologist Christian Bohr who discovered it in 1904, is a fascinating phenomenon. It explains that when the carbon dioxide levels in the blood increase or the blood becomes more acidic, hemoglobin tends to let go of its oxygen molecules. To put it simply, it’s like a signal for hemoglobin to release oxygen.
The Mechanism Behind

The Bohr Effect occurs mainly due to changes in blood pH, which refers to the concentration of hydrogen ions (H+) in the blood. When the blood becomes more acidic (in other words, when the pH decreases), there is an increase in H+ ions. These ions interact with the amino acids of hemoglobin, leading to a decrease in its affinity for oxygen and an increased likelihood of releasing the oxygen it carries.
Now, what causes the blood to become more acidic? One of the primary factors is an increase in the partial pressure of carbon dioxide. Carbon dioxide can react with water present in the blood, resulting in the formation of carbonic acid.
This acid then dissociates into H+ ions and bicarbonate ions. The enzyme carbonic anhydrase facilitates this reaction. Consequently, higher levels of carbon dioxide lead to increased carbonic acid formation, resulting in greater amounts of H+ ions and a subsequent decrease in blood pH.
As per Andrew Benner, in the quest for biochemical balance, carbonic acid undergoes a partial and reversible separation into hydrogen ions and its corresponding base, bicarbonate (HCO3). This process results in an increased availability of H+ ions in the blood, which ultimately leads to a reduction in the pH level of the surroundings.
The Significance of the Bohr Effect

The Bohr Effect is truly a captivating physiological phenomenon, and its implications for our bodily functions are significant. It plays a crucial role in how our bodies operate, particularly in the enhanced unloading of oxygen in metabolically active peripheral tissues like exercising skeletal muscle.
When we engage in physical exercise, our muscles exert more effort and generate an increased amount of carbon dioxide. This leads to a rise in the partial pressure of carbon dioxide in our blood, resulting in a reduction of the local blood pH.
Thanks to the Bohr Effect, this process facilitates a more effective release of bound oxygen by hemoglobin as it passes through the metabolically active tissue, ultimately enhancing the delivery of oxygen.
As the tissue engages in more metabolic processes, the partial pressure of carbon dioxide rises, leading to greater reductions in local pH.
Consequently, this allows for a more substantial unloading of oxygen. The impact is particularly significant in exercising skeletal muscles, which may also release lactic acid, further contributing to the reduction of local blood pH and amplifying the benefits of the Bohr Effect.
Modulation of the Oxygen-Hemoglobin Dissociation Curve

The Oxygen-Hemoglobin Dissociation Curve is not set in stone; it can be shifted by a variety of environmental factors. Factors associated with increased peripheral tissue metabolism, such as reduced pH, increased CO2, and increased temperature, shift the curve to the right.
This reduces hemoglobin’s affinity for oxygen and thus improves oxygen unloading.
Chronic hypoxia, or long-term oxygen deficiency, increases the blood’s concentration of 2,3-DPG (2,3-diphosphoglycerate), a molecule that also shifts the curve to the right.
On the other hand, the presence of fetal hemoglobin (HbF) and carbon monoxide (CO) shift the curve to the left, increasing the oxygen affinity of hemoglobin.
For a deeper understanding of the respiratory process, consider exploring this comprehensive resource.